Durability of Concrete Soaked in Drainage Water
of Salt Accumulated Fields
Hidehiko OGATA*, Shushi SATO**, Sadahiro YAMAMOTO*,
Kyoichi OTSUKI***, Hassan KHALED** and Kunio HATTORI*
* Faculty of Agriculture, Tottori University
** United Graduate School of Agriculture, Tottori University
*** Faculty of Agriculture, Kyushu University
Summary
Most drainage canals in arid or semi-arid countries where salinity
is one of the biggest problems are unlined. To send smoothly out saline
drainage water to avoid secondary salinization, concrete-lined canals are
needed. However, concrete probably deteriorates in the saline leachate.
To investigate the possibility of the deterioration, the durability of
concrete in saline water was experimentally examined. The experiment was
conducted by soaking concrete specimens in artificial saline drainage water
based on the water quality data of drainage water in the Hetao Irrigation
Area of Inner Mongolia in China. The following results were obtained: concrete
is slightly deteriorated by the leachate; the leachate affects the part
of the concrete subjected to repeated wetting and drying, but not the soaked
part of the concrete.
Key words : drainage water, durability of concrete, leaching, salt accumulated field, soaking experiment
1. Introduction
Leaching is a common technique to reduce salinity in fields where salts have accumulated. Generally, HCO3-, NO3-, Cl- and SO4- are included as anions, and Ca2+, Mg2+, Na+ and K+ are included as a cations in the leachate (Ji et al., 1998).
Most drainage canals in arid or semi-arid countries where salinity
is one of the biggest problems are unlined. To send smoothly out saline
drainage water to avoid secondary salinization, concrete-lined canals are
needed. However, concrete probably deteriorates in the saline drainage
water. Thus the extent of the deterioration should be known to estimate
the life of a concrete drainage canal and to find anti-deterioration measures.
To investigate the possibility of the deterioration, the durability of
concrete in saline water was experimentally examined. The experiment was
conducted by soaking concrete specimens in artificial saline drainage water
based on the water quality data of drainage water in the Hetao Irrigation
Area of Inner Mongolia in China.
2. Chemical deterioration of concrete
The composition of Ordinary Portland cement is shown in Table 1.
|
Table 1 Composition of Ordinary
Portland cement (%) (Kobayashi, 2000)
|
|
3CaO・SiO2
|
2CaO・SiO2
|
3CaO・Al2O3
|
4CaO・Al2O3・Fe2O3
|
|
50
|
26
|
9
|
9
|
When cement hydrates, 3CaO・2SiO2・3H2O and Ca(OH)2 are formed. The sulfates of Na, Mg, and K react with the Ca(OH)2 and 3CaO・Al2O3 in cement to form the cement bacillus (3CaO・Al2O3・3CaSO4・32H2O) .
The large cement bacillus crystals cause increase in solid volume. As a result, the concrete is severely damaged, and might fail under stress. The destructive action of MgSO4 is more violent than that of other sulfates because of the generation of the cement bacillus reacting with Ca(OH)2 as well as because of the hydrated calcium silicate decomposition. In addition, concrete deteriorates more rapidly when a physical action like repeated wetting and drying acts synergistically with the chemical action of the sulfates. Chloride attack is less severe than that of sulfates.
The chlorides of Ca, Na and K decompose a concrete surface subjected
to repeated wetting and drying. However, if concrete quality is excellent,
chloride penetration is not an important factor in concrete deterioration
(Okada, 1986).
3. Experiments
3. 1 Soaking solution
The soaking solution used in this experiment was based on the water quality data of drainage water in the Hetao Irrigation Area of Inner Mongolia in China from 1979 to 1981 (Ji et al., 1998). The salinity composition of drainage water in the Hetao Irrigation Area is shown in Table 2. Examining the durability of concrete exposed to highly aggressive solutions would indicate the life of concrete with a factor of safety. Therefore, the soaking solution used in this experiment was made using three times the amount of the reagent calculated from Table 2.
|
Table 2 Salinity
composition of drainage water in Hetao Irrigation
Area Inner Mongolia in China (Ji et al., 1998)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Na+
|
K+
|
|
|
|
|
|
|
|
|
|
|
17.88
|
1.12
|
|
|
|
|
|
|
|
|
|
|
15.49
|
0.01
|
|
|
|
|
|
|
|
|
|
|
15.88
|
0.02
|
|
|
|
|
|
|
|
|
|
|
16.42
|
0.38
|
|
|
3. 2 Concrete specimen
The concrete specimens used in the soaking experiment were six cylinders of φ100mm×200mm and six square columns of 100mm×100mm×400mm. The concrete mixture proportions are shown in Table 3. The mould of each concrete specimen was removed the day after the concrete was placed. Concrete specimens were cured in a water tank at 20℃ for 14 days. The concrete specimens were then placed in the Arid Land Dome, Arid Land Research Center of Tottori University, where they were soaked in the solution.
|
Table 3 Concrete mixture proportions
|
|
Type of cement
|
Maximum size of aggregate (mm)
|
Slump
(cm)
|
Air
(%)
|
Water-cement ratio (%)
|
Sand percentage (%)
|
Unit (kg/m3)
|
|
Water
|
Cement
|
Fine aggregate
|
Coarse
aggregate
|
|
Ordinary Portland
cement
|
20
|
12
|
2.0
|
55
|
48.2
|
206
|
374
|
819
|
914
|
3. 3 Soaking method and measurement item
Three different tests were conducted for soaking the concrete specimens,
for the whole specimen, for the half length of the specimen, and for the
half thickness of a specimen. Soaking tests for the half length and thickness
of the specimens were conducted to examine the variation in deterioration
due to differences in the dry to wet ratio. Volume change ratio, density,
ultrasonic pulse velocity and dynamic modulus of elasticity of each concrete
specimen were measured at intervals of one month.
4. Results
In this paper, only the results for the cylindrical specimens are shown. The results of the cylindrical specimens and the square column specimens were almost identical. Volume change ratio, density, ultrasonic pulse velocity and dynamic modulus of elasticity of the cylindrical specimens are shown in Fig. 1 to Fig. 4. Change in pH of the soaking solution is shown in Fig. 5, and change in EC of the soaking solution is shown in Fig. 6.
 |
|
 |
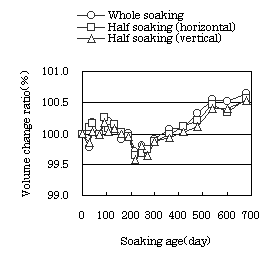
| Fig. 1 Volume change ratio of cylindrical specimens |
|
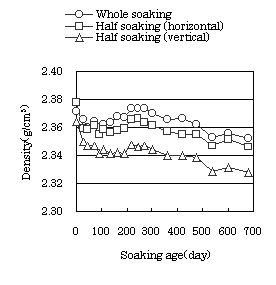
Fig. 2 Density of cylindrical specimens |
 |
|
 |
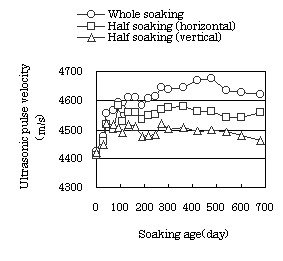
| Fig. 3 Ultrasonic pulse velocity of cylindrical specimens |
|
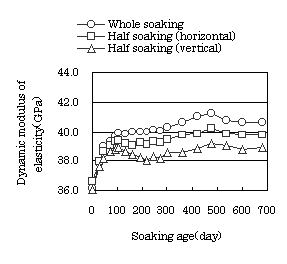
Fig. 4 Dynamic modulus of elasticity of cylindrical specimens |
 |
|
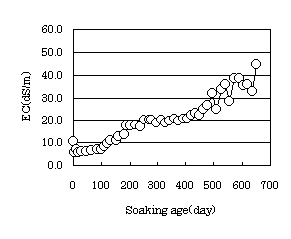 |
Fig. 5 Change of pH in soaking solution
|
|
Fig. 6 Change of EC in soaking solution |
The volume change ratio increased until soaking age of 100 days, then
decreased until soaking age of 200 days. After 200 days, the ratio increased
remarkably. The density of the concrete decreased until soaking age of
100 days, then increases until soaking age of 200 days. After 200 days,
the density decreases remarkably. The ultrasonic pulse velocity and the
dynamic modulus of elasticity increased until soaking age of 100 days,
then decreased until soaking age of 200 days, then decreased during increase
in the soaking age to 500 days. Thus, the rate of change in each variable
changed at the time of 100, 200 and 500 days. This is probably due to the
complex relationship between various compounds in the soaking solution.
It is necessary to verify these results by further research.
On the comparison of the volume change ratio, density, ultrasonic pulse
velocity and dynamic modulus of elasticity for the different soaking conditions,
whole soaking gave the largest values in any variable, and soaking half
length of the specimen gave the smallest. These results could be caused
by the difference in the supply of the curing water or the total evaporation
in each case.
The soaking solution was replenished as it decreased in volume, but did not perfectly replaced. The pH value was stable at about 9. The EC suggested the tendency to increase with soaking age. Increase in EC indicates that the density of the solution increases due to evaporation of the soaking solution, but not from the influence of the mixed materials in the concrete. The increase in EC gave a suitable result for the physical properties of the soaking specimen.
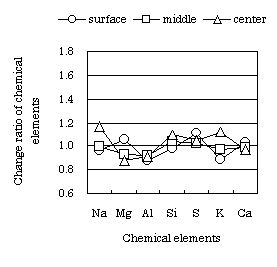
The change ratio of a chemical element of the inner concrete at the soaking age 679 days is shown Fig. 7 and Fig. 8. The chemical element was analyzed by fluorescent X-ray analysis. The chemical element was compared with a standard cured concrete specimen. Fig. 7 is the change ratio of a chemical element located below the water surface of the specimen with half length soaking. Fig. 8 is the change ratio of a chemical element located above the water surface. Both figures show the change ratio of a chemical element at three points: the surface of the specimen, midway between the surface and center, and at the center. The center is at 5cm depth from a surface.
 |
|
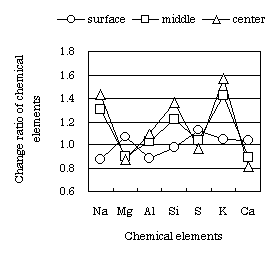 |
Fig. 7 Change ratio of chemical element in concrete located belowthe water
surface
(soaking age : 679 days) |
|
Fig. 8 Change ratio of chemical element in concrete located abovethe water surface
(soaking age : 679 days) |
The change ratio of a chemical element in concrete located below the
water surface is small, and the soaking solution does not affect the internal
concrete. On the other hand, the change ratio of a chemical element in
concrete located above the water surface is large, and the soaking solution
has affected the internal concrete. It is understood that Na and K in solution
penetrate concrete located above the water surface due to capillarity rise,
and Na and K are concentrated by repeated wetting and drying. The soaking
solution did not affect the soaked part of the concrete, but affects the
part of the concrete subjected to repeated wetting and drying. However,
even when Na and K penetrate the concrete, the concrete quality is not
greatly deteriorated by a soaking solution that includes substantial chlorides.
The measured concrete deterioration from Na and K after 500 days soaking
is slight.
5. Discussion
Deterioration and the expansion of the cement hydration by the soaking
solution are seen from an increase in volume change ratio and decrease
in density after 200 days soaking. It is also understood that the specimen
deteriorates little by little because of the erosion and expansion of cement
hydration. However, the existence of deterioration cannot be verified by
the measurements of the ultrasonic pulse velocity and the dynamic modulus
of elasticity. Deterioration in the soaking experiment occurred at the
surface of the concrete specimens. With mildly aggressive solutions like
the leachate, the physical properties of concrete are not greatly deteriorated
even if Na and K penetrate the concrete. Therefore, it is necessary to
conduct considerably long-term experiments in order to diagnose deterioration
using ultrasonic pulse velocity and dynamic modulus of elasticity, which
evaluate physical properties at the center of the specimen.
In Japan, the standard for concrete materials is strict, and the required
quality is considerably high. In this experiment used Japanese materials.
It will be necessary to repeat the experiment using materials from the
country where the salt accumulation problem is actually occurring. If low-quality
material is used, the concrete will surely be deteriorated by the leachate.
To overcome this deterioration, measures such as the addition of fly ash
to the concrete will be necessary.
6. Conclusions
The results of this research are as follows:
(1) Concrete is lightly deteriorated by the leachate in the Hetao Irrigation Area of Inner Mongolia in China.
(2) Deterioration and the expansion of the cement hydrate by the soaking
solution are seen from the increase in volume change rate and the decrease
in density after 200 days of soaking.
(3) The leachate does not affect the soaked part of concrete, but affects
the part of concrete subjected to repeated wetting and drying, because
the repeats concentrate Na and K solutions.
(4) In the soaking experiment on concrete in leaching drainage water,
deterioration of concrete can be found by soaking half length of the specimen.
(5) Long-term experiments can be required to diagnose deterioration using
ultrasonic pulse velocity and dynamic modulus of elasticity, which can
evaluate physical properties in the center of the specimen.
Acknowledgement
The authors wish to thank Dr. Masayoshi HARADA and Mr. CHERIF Ould Ahamed of Faculty of Agriculture, Tottori University for valuable advice and the help with respect to the experiment.
References
Ji, B., K. Otsuki, T. Akae and K. Nagahori : Water Management and Regional
Environment in the Hetao Irrigation Area Inner Mongolia, China, Jour. the
Japanese Society of Irrigation, Drainage and Reclamation Engineering, 66(8)
7-11 (1998) in Japanese
Okada, K. : Durability of Concrete, Asakura-Shoten, Tokyo(1986) in Japanese
Kobayashi, K. : Concrete Engineering, Shinpoku-Shuppan, Tokyo(2000) in
Japanese